In the realm of winter wonder, snowflakes exemplify nature’s artistry, each unique with formations of their own. These delicate, symmetrically intricate frozen masterpieces have urged scientists to raise a multitude of questions about their diversity and the journeys they take from the sky to the Earth’s surface. To the naked eye, a mound of snow is a fluffy, frozen pile of fun, but how does each individual snowflake form? How are they, in fact, so perfectly shaped? These questions can only be answered if we peek inside the microscopic world of snowflakes.
Although snowflakes have numerous, fascinating properties, how have scientists developed the methods to study them? The answer lies in a simple microscope and meticulous attentiveness. The first scientist to take detailed images of a snowflake was Wilson A. Bentley. This snowflake pioneer spent a majority of his life sitting in the snow, patiently waiting for snowflakes to land on a feather where he could then observe the snowflake. Interestingly, Bentley created his own device that could take closeups of snowflakes; he attached a microscope to the end of his camera. Over the course of his career, he took thousands of photos of snowflakes, giving him the nickname “Snowflake Bentley”. Similarly, in modern times, scientists still use camera and microscope conjunctions; however, the technology is simply elevated to a level incomparable to Bentley’s contraption. Recently, Nathan Myhrvold, a photographer and scientist, created his own device that can take the highest-resolution photographs of snowflakes worldwide. By combining high technology cameras and microscopes with an extremely fast flash (one-millionth of a second) with an artificial sapphire slide that could preserve the snowflake, Myhrvold created history.
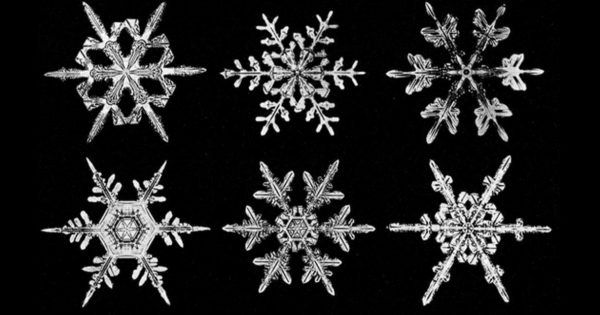
The birth of a snowflake begins with the presence of water vapor in the atmosphere. As the temperature drops, the water vapor freezes onto particles in the air, usually microscopic dust or pollen, which becomes the core of the crystal. Overtime, the crystal grows as water adheres to its surface. Certain humidities and temperatures make the flat surfaces of the crystal become unstable by the Mullins-Sekerka instability property. This property causes even small bumps on the crystal to become pronounced much faster than any other nearby areas, and once an irregularity like that becomes flat enough, it then repeats the Mullins-Sekerka instability property to create smaller bumps attached to the larger one. This repeated process creates a fern-like shape known as a dendrite. Varying atmospheric conditions result in a multitude of dendrite patterns, especially considering how sensitive the branching patterns are to miniscule changes in temperature or humidity. This is the origin of the common saying that no two snowflakes are the same. In general, warmer temperatures lead to snowflakes being more sticky. This allows more water to adhere to the branches of the snowflake, allowing simpler but larger designs of snowflakes to be produced. Conversely, colder temperatures lead to more sophisticated designs of snowflakes such as dendrite snowflakes. Humidity also plays a key role in the shaping of a snowflake, as drier weather slows the growth of snowflakes leading to more intricate shapes, while humid weather does just the opposite.
While the formation of snowflakes is precise and complicated, the symmetry of these snow crystals can be explained quite simply. In a Scientific American article written by Miriam Rossi, a professor of chemistry at Vassar College, snowflakes “reflect the internal order of the water molecules as they arrange themselves in the solid state (the process of crystallization).” On a molecular level, water in its solid state forms fragile hydrogen bonds between each water molecule in a hexagonal, symmetrical structure. As water molecules collide, they have equal probability of colliding on any side of the given hexagonal crystal. This leads to a rectangular shape of a crystal, however, the longer side will have higher probability of hydrogen bonding with other water molecules, and so as more water molecules bind to the crystal, the shape becomes hexagonal again. Overtime, this process continues and the snowflake begins to grow. While the process of a growing snowflake is slightly complicated, the branching arms of the snowflake is quite simple. As the hexagonal snowflake grows, the vertices have higher probability of condensation with colliding water molecules due to their seclusion from the rest of the snowflake, resulting in an elongation of the vertices, hence, the branches of the snowflake. From there, as the snowflake begins to develop, the overall formation follows the same structure disregarding the effects of humidity and temperature differences.

Whereas individual snowflakes may differ in their patterns and formations, all snowflakes are similar in their symmetrical natures. However, snowflakes can be divided into many categories based on their formation; the most common of these being hexagonal plates, stellar plates, dendrites, needles, and columns. Hexagonal plates generally have a flat, six-sided crystal structure and resemble tiny circular discs. These types of snowflakes tend to be the most common type of snowflake and develop during milder temperatures. A contrastingly similar type of snowflake are stellar plates. These flakes have intricate branches extending from a basic, flat hexagonal structure which develop during high-humidity conditions. Dendrites too have intricate, tree branch like designs growing out from their central structure concluding they form in cold, humid environments. These three snowflake types are the most common, frequently seen throughout the world, but other types of snowflakes, namely columns and needle snowflakes, stand out for their unique formations. Column snowflakes are known for their “elegance”, having a slender, elongated crystal shape, often seen in colder environments. Lastly, needle snowflakes have an appearance like ice splinters, and are also frequently formed in cold temperatures, characterized by light and fluffy snowfalls.

The sophisticated beauty of snowflakes has attracted scientists for hundreds of years. As our chemical knowledge and imaging technology has developed, scientists have finally gained a strong understanding of the beginning, life duration, and end of snowflake variations. Interestingly, the studies on snowflakes, and information about symmetry and patterns, can also be applied to many fields such as mathematics. For example, symmetric properties of specific problems can significantly reduce the complexity of calculations, allowing clearer visualization of pathways that may lead to the solution. Ultimately, these snow crystals are a complex masterpiece created by nature, presenting a form of symmetry and variety through the conversion of water vapor into a unique snowflake.